Scientists attempt to come up with treatments for diabetes by trying many different options. One of those is using stem cells to restore the insulin-producing cells that are being destroyed by the body's own immune system in type 1 (but not type 2) diabetes. This is being done to restore the normal body metabolism, in which insulin regulates the uptake of glucose by cells, and keeps it in balance. An important process, as without proper regulation of our body's glucose, which is used as fuel, increasing concentrations can become harmful and eventually lethal. At the Columbia University Medical Center, an attempt is being made to restore insulin production in patients with type 1 diabetes by trying to get the gut to produce it. Cells from the intestine are supposedly able to take over this function, and it may help therefore help to counter the disease with body-own assets.
Progenitors
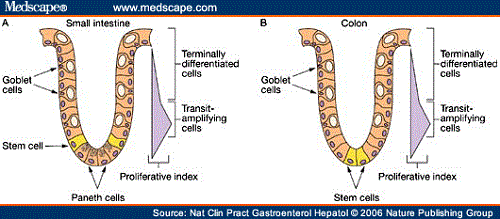
In the gut, a constant production of new cells is needed, because the lining only lasts for a short period of time. This most inner layer of the intestines is tasked with providing a wall for all the food components that come by, and selectively taking up that what is needed. Deeper inside the gut, there are more primitive cells that provide the required renewal of the gut lining. So-called progenitor cells have the capability to divide and create daughter cells, which can specialize in the type of gut cells that are required at a certain point in time. They are already of great interest to scientists, because we are able to manipulate them into becoming cells of our choice, helping us to restore organs in disease.
Foxo1
Progenitor cells suddenly become a lot more interesting to diabetes patients when a gene called Foxo1 is turned off. Then, an insulin-producing cell is added to the arsenal of cells that such progenitors are able to specialize into. Scientists at the Columbia University Medical Center turned this particular gene off in mice, and found that even in adults, that have already 'completed' the development phase, it is still possible to reprogram them to supply insulin-producing cells. Gut-derived producers also seem to be doing their job quite well: they release insulin to the bloodstream and appeared to be able to lower the concentration of glucose in diabetic mice to near-normal levels.
Therapy
Next is of course attempting to get this to work in humans by providing a viable therapy. It is an interesting new option, because other methods involve transplantation of tissue or getting stem cells to do the work. However, transplantation faces the problem of rejection, and it is still not possible to finetune stem cells in such a way that their insulin production keeps the body metabolism in balance. In some cases, reprogrammed stem cells even produce insulin when the body does not need it, which can result in dangerously low glucose levels. Reprogrammed gut cells seem to be more finely adjusted for producing insulin.
Outlook
The fact that gut cells seem to provide a replacement for normal insulin-producing cells is nothing short of amazing. Why the primitive cells that are normally tasked with resupplying the specialized gut structures can be coaxed into producing insulin by simply turning off one gene is not known. What is even more baffling is that turning this gene off in the pancreas, where the insulin-producing cells are normally found, does absolutely nothing. Reprogramming the gut seems to be a viable new option for restoring insulin production in type 1 diabetes, if we can make a working therapy from it. Patients with type 2 diabetes will not benefit from it, however. In this form of the disease, cells have become desensitized, which results in insulin not being able to do its job anymore, despite its presence.
Progenitors
 |
| Villi = inner lining of the gut. Large surface area for uptake of food. Progenitors are in the crypts, above the muscle layers. |
Foxo1
Progenitor cells suddenly become a lot more interesting to diabetes patients when a gene called Foxo1 is turned off. Then, an insulin-producing cell is added to the arsenal of cells that such progenitors are able to specialize into. Scientists at the Columbia University Medical Center turned this particular gene off in mice, and found that even in adults, that have already 'completed' the development phase, it is still possible to reprogram them to supply insulin-producing cells. Gut-derived producers also seem to be doing their job quite well: they release insulin to the bloodstream and appeared to be able to lower the concentration of glucose in diabetic mice to near-normal levels.
Therapy
Next is of course attempting to get this to work in humans by providing a viable therapy. It is an interesting new option, because other methods involve transplantation of tissue or getting stem cells to do the work. However, transplantation faces the problem of rejection, and it is still not possible to finetune stem cells in such a way that their insulin production keeps the body metabolism in balance. In some cases, reprogrammed stem cells even produce insulin when the body does not need it, which can result in dangerously low glucose levels. Reprogrammed gut cells seem to be more finely adjusted for producing insulin.
Outlook
The fact that gut cells seem to provide a replacement for normal insulin-producing cells is nothing short of amazing. Why the primitive cells that are normally tasked with resupplying the specialized gut structures can be coaxed into producing insulin by simply turning off one gene is not known. What is even more baffling is that turning this gene off in the pancreas, where the insulin-producing cells are normally found, does absolutely nothing. Reprogramming the gut seems to be a viable new option for restoring insulin production in type 1 diabetes, if we can make a working therapy from it. Patients with type 2 diabetes will not benefit from it, however. In this form of the disease, cells have become desensitized, which results in insulin not being able to do its job anymore, despite its presence.

No comments:
Post a Comment